SIEF - Registration 2013
You have to register your substance before 1st June, 2013 if:
- You produce or import phase-in substance in the quantity reaching 100 t/a or more;
- Your substance is not classified as CMR, categories 1 or 2, or R 50/53;
- You pre-registered the substance before 1st December, 2008.
Registration 2013
>>
SIEF - communication
User friendly, advanced SIEF-IT solutions including wide range of software developments for hundreds of potential registrants to enable effective cooperation and communication in compliance with REACH regulation.
SIEF - data sharing
Safe and reliable, SIEF-IT helps hundreds of pre-registrants of the same substance to share information about the substance and to avoid duplication of expensive testing…
Check QSAR eye-irritation endpoints
The system is designed for Producers and Importers who want to follow the REACH regulation and whose priority is to minimize the costs of registration dossier preparation and costs of registration in ECHA.
You are welcome to browse data based on computational QSAR-Quantitative Structure Activity Relationship models. The offer includes biological, toxicological and physicochemical endpoints for more than 30000 compounds. All the models are in accordance with criteria worked out by the OECD QSAR Ad Hoc Group and are ready for dossier submission to IUCLID5.
SIEF LoA (Letter of Access)
SIEF-IT system enables communication between Lead Registrant and SIEF members regarding distribution of LoA:
- Lead Registrant – you can add your LoA for sale;
- SIEF member - can purchase LoA in agreement with Lead Registrant.
There is not much time …
Please note that certain tests mentioned in Appendix IX of REACH regulation, required during dossier submission in ECHA, are very long-lasting...
Renowned laboratories applying GLP (Good Laboratory Practice) rules, authorised to provide tests for dossier preparation, estimate time required for test preparation, for example ecotoxicological, for at least 6 months for one trial.
Therefore, it is very important to start your dossier preparation long before the deadline.
There are two main pillars of SIEF-IT, designed to help on your way to ECHA registration.
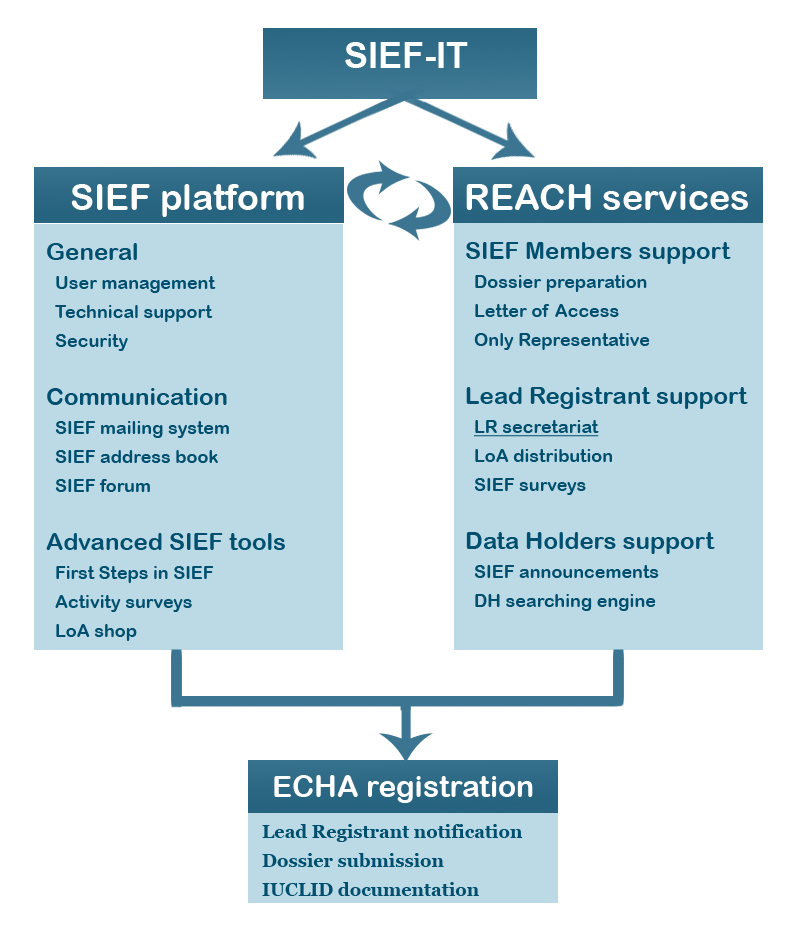 SIEF platform and REACH services - two pillars of SIEF-IT.
Expand to complete list of SIEF support
SIEF platform and REACH services - two pillars of SIEF-IT.
Expand to complete list of SIEF support
| SIEF platform |
REACH services |
ECHA registration |
General:
- Possibility of SIEFs formation
- Easy joining procedure
- Security
- User account creation
- User subaccounts management
- Login data recovery
- Technical support
- XML import from REACH-IT
Basic SIEF tools:
- Inviting pre-SIEF members
- LR searching engine
- DH searching engine
- QSAR searching engine
- Letter of Access
Communication:
- SIEF mailing system
- Pre-SIEF address book
- SIEF address book
- LR&SFF subforums
- Substance subforums
- Data Holders’ subforums
- Experts subforums
- Quick messages to pre-SIEF
Advanced SIEF tools:
- First Steps module
- SIEF Activity Survey
- SIP survey
- SIEF subscription
- SIEF tasks module
|
SIEF Members support:
- Chemical safety report
- Exposure scenarios
- Chemical safety assessment
- IUCLID
- Letter of Access
- Only Representative
- Registration in ECHA
Lead Registrant Support:
- LR secretariat
- LoA distribution
- SIEF Activity surveys
- LR nomination – upon survey
- Availability of tests on vertebrates – upon survey
- Agreement on classification and labelling – upon survey
- All survey results in a transparent format
- Communication network
- QSAR searching engine
- SIEF announcements
- Special IT platform
- LR searching engine
Data Holders / REACH Experts support:
- SIEF announcements
- Data Holders’ searching engine
|
Registration support:
- Lead Registrant notification
- Dossier submission
- IUCLID documentation
|
 SIEF platform and REACH services - two pillars of SIEF-IT.
SIEF platform and REACH services - two pillars of SIEF-IT.
